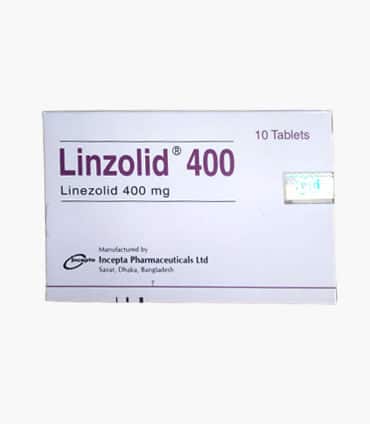
Linzolid 400 | Tablet | 1 pcs
৳ 60.00
Brand Name: Linzolid Tablet
Generic: Linezolid
400 mg
Manufacturer: Incepta Pharmaceuticals Ltd.
Unit Price: ৳ 60.00 (1 x 10: ৳ 600.00)
Strip Price: ৳ 600.00
Indications
Vancomycin-Resistant Enterococcus faecium infections including cases with concurrent bacteremia.
Nosocomial pneumonia caused by Staphylococcus aureus (methicillin-susceptible and -resistant strains) or Streptococcus pneumoniae (including multi-drug resistant strains). Combination therapy may be clinically indicated if the documented or presumptive pathogens include Gram-negative organism.
Complicated skin and skin structure infections, including diabetic foot infections (without concomitant osteomyelitis) caused by Staphylococcus aureus (methicillin-susceptible and methicillin-resistant strains), Streptococcus pyogenes or Streptococcus agalactiae.
Uncomplicated skin and skin structure infections caused by Staphylococcus aureus (methicillin-susceptible only) or Streptococcus pyogenes.
Community-acquired pneumonia caused by Streptococcus pneumoniae (including multi-drug resistant strains) including cases with concurrent bacteremia or Staphylococcus aureus (methicillin-susceptible strains only).
Pharmacology
Dosage
Patients who commence treatment on the parenteral formulation may be switched to oral presentation when clinically indicated. In such circumstances, no dose adjustment is required as Linezolid has an oral bioavailability of approximately 100%. The injection should be administered over a period of 30 to 120 minutes. The film-coated tablets or oral suspension may be taken with or without food.
Complicated skin and skin structure infections, Community-acquired pneumonia, including concurrent bacteremia-
- Pediatric Patients (Birth through 11 Years of Age): 10 mg/kg IV or oral t.i.d.
- Adults and Adolescents (12 Years and Older): 600 mg IV or oral b.i.d.
- Recommended Duration of Treatment (consecutive days): 10 to 14
Nosocomial pneumonia, Vancomycin-resistant Enterococcus faecium infections including concurrent bacteremia-
- Pediatric Patients (Birth through 11 Years of Age): 10 mg/kg IV or oral t.i.d.
- Adults and Adolescents (12 Years and Older): 600 mg IV or oral b.i.d.
- Recommended Duration of Treatment (consecutive days): 14 to 28
Uncomplicated skin and skin structure infections-
- Pediatric Patients (Birth through 11 Years of Age): <5 yrs: 10 mg/kg oral t.i.d. 5-11 yrs: 10 mg/kg oral b.i.d
- Adults and Adolescents (12 Years and Older): Adults: 400 mg oral b.i.d. Adolescents: 600 mg oral b.i.d
- Recommended Duration of Treatment (consecutive days): 10 to 14
Neonates <7 days: Most pre-term neonates <7 days of age (gestational age <34 weeks) have lower systemic Linezolid clearance values and larger AUC values than many full-term neonates and older infants. These neonates should be initiated with a dosing regimen of 10 mg/kg every 12 hours. Consideration may be given to the use of 10 mg/kg in every eight hours regimen in neonates with a sub-optimal clinical response. All neonatal patients should receive 10 mg/kg t.i.d. by 7 days of life.
Administration
Reconstitution of Oral Suspension: Shake the bottle to loosen powder. Add 75 ml (with the help of given cup) of boiled and cooled water to the dry mixture in the bottle. For the ease of preparation, add water to the bottle in two portions. Shake well after each addition until all the powder is in suspension.
Note: Shake the suspension well before each use. Keep the bottle tightly closed. The reconstituted suspension should be stored in a cool and dry place. Use within 21 days after constitution.
Intravenous Administration: Linezolid IV Injection is supplied in single-use, ready-to-use infusion bottles. Linezolid IV Injection should be administered by intravenous infusion over a period of 30 to 120 minutes. The intravenous infusion bottles should not be used in series connections. Additives should not be introduced into this solution. The infusion bottles should be stored at room temperature and protected from freezing. Linezolid IV Injection may exhibit a yellow color that can intensify over time without adversely affecting potency.
Interaction
Monoamine Oxidase Inhibition: Linezolid is a reversible and nonselective inhibitor of monoamine oxidase. Therefore, Linezolid has the potential for interaction with adrenergic and serotonergic agents.
Adrenergic Agents: Some individuals receiving Linezolid may experience a reversible enhancement of the pressor response to indirect-acting sympathomimetic agents, vasopressor or dopaminergic agents. Initial doses of adrenergic agents such as dopamine or epinephrine should be reduced and titrated to achieve the desired response.
Serotonergic Agents: Physicians should be alert to the possible signs and symptoms of serotonergic syndrome in patients receiving concomitant Linezolid and serotonergic agents.
Contraindications
Side Effects
Pregnancy & Lactation
Precautions & Warnings
Overdose Effects
Therapeutic Class
Storage Conditions
| Generic Name | Linezolid |
|---|---|
| Tablet | 400 mg |
Only logged in customers who have purchased this product may leave a review.






Reviews
There are no reviews yet.