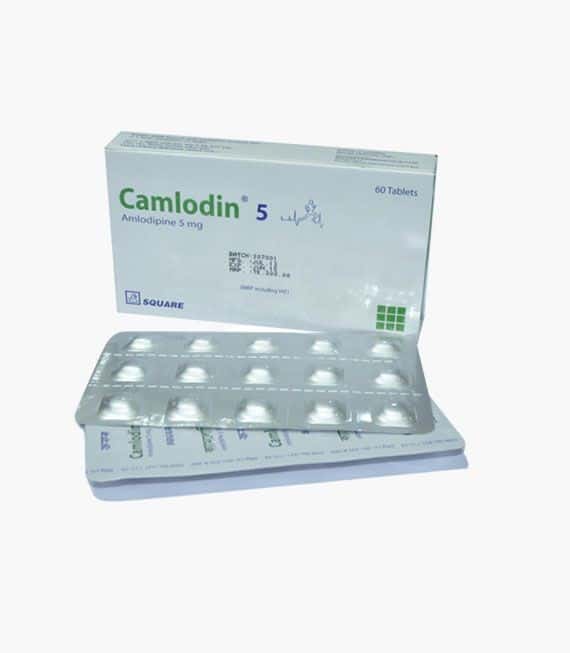
Camlodin 5 | Tablet | 10 pcs
৳ 50.20
Brand Name: Camlodin Tablet
Generic: Amlodipine
5 mg
Manufacturer: Square Pharmaceuticals Ltd.
Unit Price: ৳ 5.02 (60’s pack: ৳ 301.20)
Indications
Hypertension: Amlodipine is indicated for the treatment of hypertension. It may be used alone or in combination with other antihypertensive agents.
Stable angina: Amlodipine is indicated for the treatment of chronic stable angina. It may be used alone or in combination with other antianginal agents.
Vasospastic angina: Amlodipine is indicated for the treatment of confirmed or suspected vasospastic angina. The drug may be used as single therapy or in combination with other antianginal drugs.
Therapeutic Class
Pharmacology
Amlodipine is a calcium ion influx inhibitor of the dihydropyridine group (slow channel blocker or calcium ion antagonist) and inhibits the transmembrane influx of calcium ions into cardiac and vascular smooth muscle.
The mechanism of the antihypertensive action is due to a direct relaxant effect on vascular smooth muscle. The precise mechanism by which amlodipine relieves angina has not been fully determined but Amlodipine reduces total ischemic burden by the following two actions:
1. Amlodipine dilates peripheral arterioles and thus, reduces the total peripheral resistance (afterload) against which the heart works. Since the heart rate remains stable, this unloading of the heart reduces myocardial energy consumption and oxygen requirements.
2. The mechanism of action of Amlodipine also probably involves dilatation of the main coronary arteries and coronary arterioles, both in normal and ischemic regions. This dilation increases myocardial oxygen delivery in patients with coronary artery spasm (prinzmetals or variant angina).
Dosage & Administration
Interaction
Contraindications
Amlodipine is contraindicated in patients with–
1. Hypersensitivity to amlodipine, dihydropyridine derivatives or any of the excipients
2. Shock (including cardiogenic shock)
3. Obstruction of the outflow-tract of the left ventricle (e.g. high grade aortic stenosis)
4. Unstable angina
5. Hemodynamically unstable heart failure after acute myocardial infarction (during the first 28 days)
6. Severe hypotension
Side Effects
Pregnancy & Lactation
Pregnancy Category C. The safety of amlodipine in human pregnancy has not been established. In animal studies, reproductive toxicity was observed at high doses. Use in pregnancy is only recommended when there is no safer alternative and when the disease itself carries greater risk for the mother and fetus.
Lactation: It is not known whether amlodipine is excreted in breast milk. A decision on whether to continue/discontinue breast-feeding or to continue/discontinue therapy with amlodipine should be made taking into account the benefit of breast-feeding to the child and the benefit of amlodipine therapy to the mother.
Precautions & Warnings
Hypotension: Since the vasodilatation induced by Amlodipine is gradual in onset, acute hypotension has rarely been reported after oral administration of Amlodipine. Nonetheless, caution should be exercised when administering the drug with any other peripheral vasodilator particularly in patients with severe aortic stenosis.
Cardiac failure: Patients with heart failure should be treated with caution. Calcium channel blockers, including Amlodipine, should be usedwith caution in patients with congestive heart failure, as they may increase the risk of future cardiovascular events and mortality.
Beta blocker withdrawal: Amlodipine gives no protection against the danger of abrupt beta blocker withdrawal; any such withdrawal should be gradualreduction of the dose of beta blocker.
Hepatic failure: The half-life of amlodipine is prolonged and AUC values are higher in patients with impaired liver function. Amlodipine should therefore be initiated at the lower end of the dosing range and caution should be used, both on initial treatment and when increasing the dose. Slow dose titration and careful monitoring may be required in patients with severe hepatic impairment.
Use in Special Populations
Children with hypertension from 6 years to 17 years of age: 2.5 mg once daily as a starting dose, up-titrated to 5 mg once daily if blood pressure goal is not achieved after 4 weeks. Doses in excess of 5 mg daily have not been studied in pediatric patients.
Children under 6 years old: The effect of amlodipine on blood pressure in patients less than 6 years of age is not known.
Elderly: Amlodipine used at similar doses in elderly or younger patients is equally well tolerated. Normal dosage regimens are recommended in the elderly, but increase of the dosage should take place with care.
Renal impairment: Changes in amlodipine plasma concentrations are not correlated with degree of renal impairment, therefore the normal dosage is recommended. Amlodipine is not dialysable.
Hepatic impairment: Dosage recommendations have not been established in patients with mild to moderate hepatic impairment; therefore dose selection should be cautions and should start at the lower end of the dosing range. The pharmacokinetics of Amlodipine have not been studied in severe hepatic impairment. Amlodipine should be initiated at the lowest dose (2.5 mg once daily) and titrated slowly in patients with severe hepatic impairment.
Overdose Effects
Storage Conditions
| Generic Name | Amlodipine |
|---|---|
| Size | 5 mg |
Only logged in customers who have purchased this product may leave a review.




Reviews
There are no reviews yet.